Enzymatic Inhibition Constant of Acetylcholinesterase for the Electrochemical Detection and Sensing of Chlorpyrifos
Main Article Content
Abstract
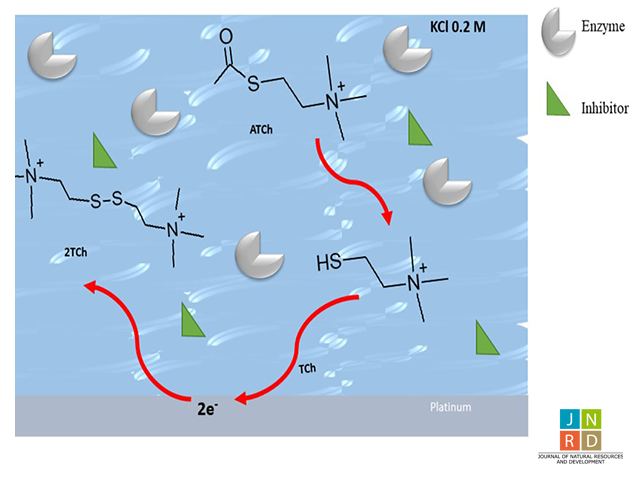
Infiltration into soils of pesticides used during agricultural production has led to the contamination of aquatic ecosystems due to their long persistence in the environment. Some pesticides (e.g. Chlorpyrifos) are inhibitors of cholinesterase enzyme activity and their presence in water samples can be indirectly detected by a decrease in enzymatic activity. Biosensors based on cholinesterase enzymes are an alternative for the sensitive detection of important contaminants in the environmental sector. Acetylcholinesterase enzyme (AChE) catalyzes the hydrolysis of acetylthiocholine (ATCh) to produce thiocholine (TCh). This feature can be employed to measure the decrease in AChE activity. The inhibitory characteristics of the AChE-Chlorpyrifos system have been studied through cyclic voltammetry, by evaluation of the oxidation of the thiol group, which corresponds to TCh production on platinum electrodes in the presence of an inhibitor. In the present study, enzymatic curves were obtained at different concentrations of substrate and inhibitor, which were then used to determine the enzymatic kinetics corresponding to a mixed inhibition type, with an inhibition constant (Ki) of (18.26 ± 0.01) μM. TCh electrochemical detection appears to be a promising option for the development of biosensors to identify and quantify pesticides present in the ecosystem.
Article Details
Issue
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.